Background
Most patients with follicular lymphoma (FL) have long-term disease control resulting in a prolonged overall survival (OS), beyond 20 years. However, approximately 20% of patients experience disease progression within two years after treatment initiation (POD24) which is associated with a shorter OS but early prediction of POD24 remains challenging. Positron emission tomography computed tomography (PET/CT) positivity after induction therapy has been associated with shortened progression-free survival (PFS). Also, minimal residual disease (MRD) positivity in peripheral blood using BCL2-JH translocation predicts outcome but barely 50% of patients are informative with this tumor marker. Circulating tumor DNA (ctDNA) may increase the proportion of MRD informative patients up to 80%. Our objective was to evaluate the predictive value of ctDNA for PFS and POD24, either alone or coupled with PET/CT response in first-line patients enrolled in the RELEVANCE trial.
Methods
This ancillary study was carried out on patients included by French LYSA centers in RELEVANCE, a randomized phase III trial comparing the chemotherapy-free regimen R2 versus standard R-chemo followed by Rituximab maintenance in previously untreated patients with FL. All patients who had a PET/CT at diagnosis and at week 24 (W24, end of induction treatment) and for whom serum was stored at these 2 time points were eligible. Cell-free DNA (cfDNA) was extracted from a median of 2.8 mL of serum (range 0.35 to 4.1). Libraries were generated from 129kb captured-DNA custom panel (Agilent, XTHS2). Variant calling and phased variant annotation were performed using Unique Molecular Identifiers (UMI). Libraries were sequenced with a median coverage of 3257 UMI. Concentrations of ctDNA were calculated from TapeStation migration system (Agilent) using 50 bp to 600 bp limits and expressed as log hGE/mL. To ensure maximum specificity in a context of non-optimal serum samples, only patients with phased variants in ctDNA at diagnosis were studied for MRD at W24. Total metabolic tumor volume (TMTV) was computed using the 41% SUVmax method and PET/CT response assessed with Lugano criteria.
Results
As PET/CT at W24 was optional in RELEVANCE, only 141 out of 581 patients enrolled were eligible. Compared to the overall RELEVANCE population, baseline characteristics were similar except for FLIPI (7.1% FLIPI 0-1 versus 15.9% respectively, p=0.013) and PFS event (29.3% versus 39.5% respectively, p=0.035).
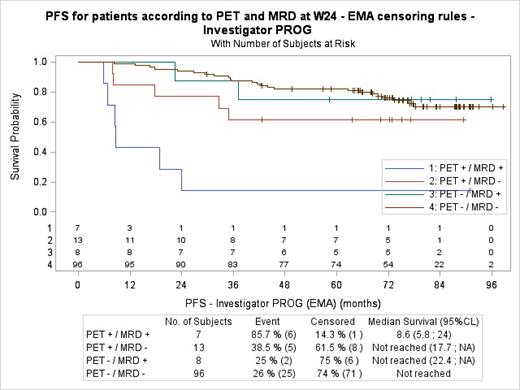
At time of diagnosis, with a median of 81 variants per patient (range 1 to 659), a mutational profile could be identified from cfDNA in 99% (140/141) of patients. A significant correlation was found between ctDNA load (median 2.39 log hGE/mL (range 1.01 to 4.20) and TMTV (median 298 mL, range 5 to 3100) (p=0.023). Phased variants were detected in 124 patients (87.9%) at diagnosis and were used for MRD analyses. At W24, ctDNA remained detectable (ctDNA+) in 15 patients, 9/69 (13%) in the R2 arm and 6/55 (11%) in the R-chemo arm (p=0.787). PET/CT remained positive (Deauville 4 and 5) in 20 patients, 11/72 (15.2%) in the R2 arm and 9/56 (16.1%) in the R-chemo arm. With a median follow-up of 6.4 years, ctDNA+ patients exhibited a median PFS of 37 months versus not reached for ctDNA- patients, p=0.0096. Similarly, PET/CT+ patients had a median PFS of 34 months versus not reached for PET/CT- patients (p<0.001). When adjusting on FLIPI in a multivariate Cox model, ctDNA remains statistically significant (HR=2.63 [1.20; 5.77], p=0.0163). Among PET/CT+ patients, 13/20 (65%) patients showed discrepant PET/CT and ctDNA responses and double positive patients (7/20, 35%) presented a shorter median PFS of 8.6 months (versus not reached, p=0.0147; Figure 1). Regarding POD24, negative predictive value (NPV) and positive predictive value (PPV) were respectively 91.7% and 46.7% for ctDNA MRD and 93.3% and 45.0% for PET/CT. When combined, PET+/MRD+ identified POD24 patients with a NPV and PPV of 91.5% and 85.7% respectively.
Conclusion
Combining the results of ctDNA and PET/CT at W24 post induction improves early prediction of POD24 in previously untreated patients with follicular lymphoma.
Disclosures
Bachy:Novartis: Honoraria, Other: Personal Fees; Incyte: Honoraria; Takeda: Honoraria; Pfizer: Honoraria, Other: Personal Fees; Hospices Civils de Lyon Claude Bernard Lyon 1 University: Current Employment; Bristol Myers Squibb: Honoraria, Other: Personal Fees, Research Funding; Amgen: Research Funding; Kite, a Gilead Company: Honoraria, Other: Personal Fees; Roche: Consultancy, Honoraria. Casasnovas:ROCHE: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; BEIGENE: Consultancy, Honoraria; GILEAD/KITE: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; MSD: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Abbvie: Consultancy, Honoraria; ADC Therapeutics: Consultancy, Honoraria; AMGEN: Consultancy, Honoraria; Astra Zeneca: Consultancy, Honoraria; TAKEDA: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Tilly:F. Hoffmann-La Roche Ltd: Honoraria, Research Funding; BMS, F. Hoffmann-La Roche Ltd, ADC therapeutics: Membership on an entity's Board of Directors or advisory committees. Cartron:Gilead: Honoraria; Roche: Consultancy, Honoraria; AbbVie: Consultancy, Honoraria; MedxCell: Consultancy; Novartis: Honoraria; Ownards Therapeutics: Consultancy; MabQi: Consultancy; Janssen: Honoraria; Emercell: Consultancy; BMS: Consultancy, Honoraria; Jansen, Gilead, Novartis, F. Hoffmann-La Roche Ltd, BMS, Abbvie: Honoraria; MedxCell, Ownards Therapeutics, MabQi, Emercell, F. Hoffmann-La Roche Ltd, BMS, Abbvie: Consultancy; MabQi, Ownards Therapeutics, Abbvie, Roche, Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees. Tessoulin:Gilead: Honoraria; Incyte: Honoraria; Abbvie: Honoraria; Kite: Honoraria. Guidez:Gilead/Kite: Honoraria; Astra-Zeneca: Honoraria; Incyte: Honoraria; Takeda: Honoraria. Cheminant:AstraZeneca: Other: Travel accomodations and Meeting inscription; Innate Pharma: Research Funding; Abbvie: Research Funding; Amgen: Honoraria. Morschhauser:F. Hoffmann-La Roche Ltd, Gilead, AbbVie: Membership on an entity's Board of Directors or advisory committees; F. Hoffmann-La Roche Ltd, AbbVie, BMS, Genmab, Gilead, Novartis: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal